The SPC manufacturing waiver has recently been adopted by European Parliament. It is still to be adopted by the Council of the European Union, although the Council is expected to do so in a matter of months. Read on to find out how and when the changes to SPC protection will affect right holders.
What are SPCs?
SPCs are a form of intellectual property that provide additional exclusivity of up to 5 and a half years beyond the normal 20-year patent term for medicinal products and agrochemicals that have obtained marketing authorisation. The rational for SPCs is to compensate right holders for the delay attributable to the market authorisation procedure. Applications for SPCs are made at each national patent office where there is a basic patent in force that protects the relevant product and where there is a relevant granted marketing authorisation.
Background to the new manufacturing waiver
A draft EU regulation amending Regulation (EC) 469/2009 (the legislation governing SPCs for medicinal products) providing an exemption for manufacturing and stockpiling activities was initially published by the European Commission in May 2018. The justification for the amendment was to allow EU-based manufacturers of generics and biosimilars to make medicines inside the EU when those medicines are intended for export to markets outside the EU, or allow them time to prepare for EU market entry as soon as possible on expiry of the SPC.
Key features of the manufacturing waiver
Manufacture of a medicinal product (or its active ingredient) will not infringe a relevant SPC if the product is intended for export to countries outside the EU.1
In addition, SPC protection will not prevent third parties from manufacturing a medicinal product or its active ingredient intended for the EU market during the last 6 months of the SPC term (to allow stockpiling prior to entry into the EU market as soon as possible after SPC expiry).2
Obligations on users of the waiver
To benefit from the waiver, a manufacturer must:
-
Provide the following information to both the national patent office that granted the SPC and the SPC proprietor 3 months prior to manufacture or the first related act:
-
The name and address of the manufacturer
-
Whether manufacture for export and/or stockpiling is intended
-
The EU member state where the export and/or stockpiling will take place
-
The SPC number
-
For exported medicinal products, the number of the marketing authorisation (or equivalent) in each country outside the EU
-
Any changes to the above before they take effect
-
For export to countries outside the EU, a new “EU export” logo must be applied to the outer packaging of the medicinal product or active ingredient.
Next legislative steps
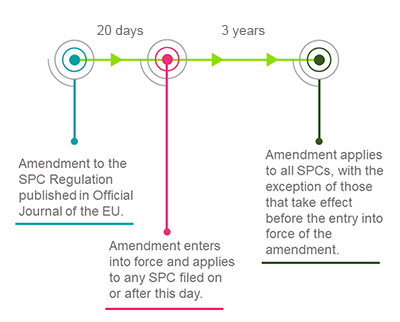
Amendments to the SPC Regulation must now be adopted by the Council of the EU, which is likely to go ahead.3 Once adopted, the regulation will enter into force on the 20th day after publication in the Official Journal of the EU (expected around June or July 2019) and will apply to all SPCs filed on or after that 20th day. After a transition period of 3 years, the waiver will also apply to SPCs with a filing date before the 20th date that take effect on or after that date.
What action can be taken now?
While the waiver is good news for manufacturers of generics and biosimilars, it will clearly curtail SPC protection.
If possible, appropriate measures should be taken to complete prosecution of pending SPC applications before entry into force of the new regulation to avoid being affected by the manufacturing waiver. In the UK, for example, accelerated examination of SPCs may be possible as reported here.
Any applicants of SPCs that are not granted by the time the amendments come into force should make sure any third-party generics or biosimilar manufacturers who intend to take advantage of the waiver comply with the obligations provided above.
If you have any questions, please contact Tom Leonard or Oliver Lam, or your usual Kilburn & Strode advisor.
1 The derogation also applies to related acts which are “strictly necessary” for making.
2 Likewise, the derogation also applies to related acts which are “strictly necessary” for stockpiling.
3 http://www.europarl.europa.eu/RegData/commissions/juri/lcag/2019/02-20/JURI_LA(2019)002691_EN.pdf