Lipid nanoparticles (LNPs) have emerged as a leading pharmaceutical formulation technology, particularly for nucleic acid delivery.
The delivery of mRNA using liposomes was first reported in 19781. In an effort to improve encapsulation of the charged mRNA, cationic lipid based LNPs2 and ionizable lipid based LNPs3 were subsequently developed. These improvements, coupled with advances in mRNA engineering, led to the development and approval of LNP-based mRNA vaccines, BNT 162b2 and mRNA-1273, during the COVID-19 pandemic.
LNPs are an attractive delivery vehicle due to their relative ease of production and modification, as well as their biocompatibility. However, numerous challenges remain for LNP clinical application, including stability, targeted delivery and endosomal escape. A particular focus is in overcoming the challenge of LNP delivery beyond the liver, the holy grail of RNA delivery, with innovation in cell and tissue-specific targeting.
Despite these challenges, several clinical trials have demonstrated success using LNPs in various therapeutic settings.
-
Onpattro® (patisiran) is an EMA approved treatment for hereditary transthyretin-mediated amyloidosis, making use of an LNP to deliver small interfering RNA (siRNA) to the liver.
-
Leqvio® (inclisiran) is an EMA approved treatment for primary hypercholesterolaemia or mixed dyslipidaemia, making use of an LNP to deliver siRNA to the liver.
-
Givlaari® (Givosiran) is an EMA approved treatment for acute hepatic porphyria, making use of an LNP to deliver the mRNA-targeting siRNA drug.
Recently, Beam Therapeutics, Inc. announced positive initial data from their ongoing clinical trial (NCT06389877) of BEAM-302 (a liver-targeting LNP formulation of a guide RNA and an mRNA encoding a base editor) in Alpha-1 Antitrypsin Deficiency (AATD), demonstrating the first ever clinical genetic correction of a disease-causing mutation.
The growing body of successful clinical trials demonstrates the versatility and effectiveness of LNPs as delivery vehicles, and we can expect to see more LNP-based products emerge in clinical practice in the coming years. Therefore, how these products are protected by IP will become of increasing importance.
Composition
All of the LNPs approved to date for clinical use comprise four lipids: ionizable cationic lipids, phospholipids (also known as a helper lipid), cholesterol, and polyethylene glycol (PEG) lipids4. Each of these components has a distinct chemical structure with specific properties that affect both safety and efficacy. The key components are the ionizable cationic lipids which have a pH-sensitive functional group. In acidic environments such as the endosome, these lipids become positively charged, facilitating disruption of the negatively charged cell and endosomal membrane, allowing release of nucleic acids into the cytosol5.
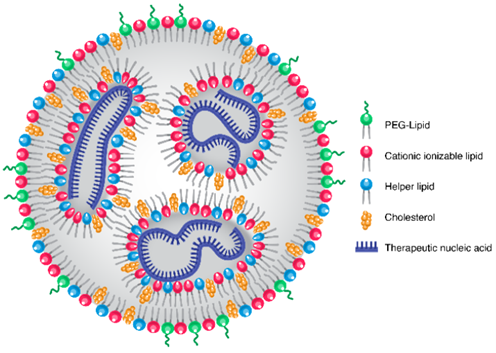
Schematic of LNP structure. Source: Kularatne et al., 20226.
Despite the general term of “LNP”, there is no universal LNP. The specific ratios, lipid types and overall composition are customised to the specific target or therapeutic purpose.
VERVE-101 and 102
The composition of LNPs can be crucial to the success of therapies. Lipids that perform well in one therapy could have critical side effects in another. This is evidenced by the pausing of Verve Therapeutics’ (hereafter “Verve”) Phase 1b clinical trial (NCT05398029) for VERVE-1017.
VERVE-101, developed by Verve, is an experimental gene-editing therapy designed to treat cardiovascular diseases, specifically to lower LDL cholesterol by editing a single base in the PCSK9 gene. VERVE-101 was given as a single intravenous infusion of an optimized adenosine base editor (ABE) mRNA and a guide RNA (gRNA) targeting PCSK9, all packaged in an LNP8.
Despite initial success, the Phase 1b trial was paused due to the discovery of a grade 3 serious adverse event (SAE) in the sixth patient to receive treatment. Verve attributed the treatment-induced increase in serum alanine aminotransferase (ALT) and thrombocytopenia to the LNP delivery system9.
As a result, Verve have since pivoted to VERVE-102 (NCT06164730), using the same ABE mRNA and gRNA for PCSK9 as VERVE-101, but a different LNP delivery system. The two principal differences being a different ionizable lipid and the incorporation of N-acetylgalactosamine (GalNAc), allowing the LNP in VERVE-102 to access hepatocytes via either asialoglycoprotein receptor (ASGPR) or low-density lipoprotein receptor (LDLR)-mediated uptake8. The GalNAc was added to enhance the efficacy and safety of the delivery method, while also making the treatment accessible to patients with homozygous familial hypercholesterolemia (HoFH).
Verve’s proprietary GalNAc-LNP is simultaneously being investigated in VERVE-20110, delivering an ABE mRNA and gRNA targeting the ANGPTL3 gene in the liver. VERVE-201 is designed to inactivate ANGPTL3 in the liver and thereby lower low-density lipoprotein cholesterol (LDL-C) concentrations to treat atherosclerotic cardiovascular disease (ASCVD).
Verve’s story highlights the importance of understanding how LNPs interact in different therapeutic scenarios. We certainly look forward to the VERVE-102 and VERVE-201 clinical trial readouts and the potential they hold.
Patent landscape
The patent landscape for LNP delivery of nucleic acids is rapidly evolving and in the case of COVID-19 vaccines, relatively litigious. The amount of innovation in this field is testament to the high value nucleic acid therapeutics market and we expect this to increase as the market continues to grow.
LNP technologies are widely protected by patents and patent applications spanning various areas of innovation. Patent applications covering LNPs generally focus on capturing the improvements in stabilisation, storage, biodistribution and targeting that this technology can achieve. Exemplary claim types include:
-
The LNP components as new chemical entities
-
Specific lipid formulations
-
Pharmaceutical formulations with the active agents
-
Methods of production
-
Methods of treatment/medical uses
There is inevitably patent overlap as advancements in formulations, targeting, and stabilisation impact different therapeutic areas. This highlights the need for innovators in the field to keep on top of the competitive landscape and in many cases seek collaborations.
As the demand for nucleic acid-based therapies continues to grow, robust IP protection will be essential in driving innovation and ensuring that these life-changing technologies reach patients effectively. Our team has considerable experience advising clients on patent matters in relation nucleic acids, LNP chemistry and LNP delivery, from obtaining global protection to freedom-to-operate diligence in what are dynamic and rapidly evolving technology areas. Please get in touch with Beth Ormrod, Benjamin Heller, or your usual Kilburn & Strode advisor if you have any related queries.
1 Ostro MJ, et al. Evidence for translation of rabbit globin mRNA after liposome mediated insertion into a human cell line. Nature. 274:921–3 (1978).
2 Malone RW, et al. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 86:6077–81 (1989).
3 Fenton OS, et al. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Adv Mater. (2016).
4 Vasileva, O. et al. Composition of lipid nanoparticles for targeted delivery: application to mRNA therapeutics. Front. Pharmacol. 15, (2024).
5 Schlich M. et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng Transl Med. 6(2):e10213 (2021).
6 Kularate, R. N. et al. The future of tissue-targeted lipid nanoparticle-mediated nucleic acid delivery. Pharmaceuticals. 15(7): 897 (2022).
7 https://www.genengnews.com/topics/genome-editing/bittersweet-symphony-verves-pause-on-verve-101-narrows-lnp-delivery-strategy/
8 https://www.vervetx.com/our-programs/verve-101-102
9 https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-announces-updates-its-pcsk9-program
10 https://www.vervetx.com/our-programs/verve-201