The development of chimeric antigen receptor (CAR) technology is a classic case of a series of incremental improvements in a technical field. It is possible to look at the structures of the different chimeric antigen receptor (CAR) generations (1st, 2nd, 3rd, 4th, and now 5th generation) and see how each successive generation builds on the previous iterations of CARs and provides a more sophisticated therapeutic approach.
The filing of patent applications in this technical field allows the use of strategies to maximise potential patent term in view of earlier filings if careful notice is taken of the requirements for patentability.
The core structure of a CAR is shown in the 1st generation CAR molecules, depicted below. These comprise the major components of the extracellular domain, the hinge, the transmembrane domain and the intracellular domain (endodomain).
The subsequent evolution of the development of CARs from the first generation, which contained only a single intracellular domain comprising an immunoreceptor tyrosine-based activation motif (ITAM) motif, to later generations with more complex intracellular structural arrangements and function shows how the CARs became more adapted to address specific issues.
Note that in traditional T cell function, in order for the αβ or γδT cell receptor chains (TCR) to integrate extracellular stimuli into the appropriate intracellular cellular response, they must utilize the immunoreceptor tyrosine-based activation motifs (ITAMs) found within the CD3 subunits CD3γε, CD3δε, and ζζ) of the TCR signaling complex. The CD3 zeta chain plays an important role in coupling antigen recognition to several intracellular signal-transduction pathways.
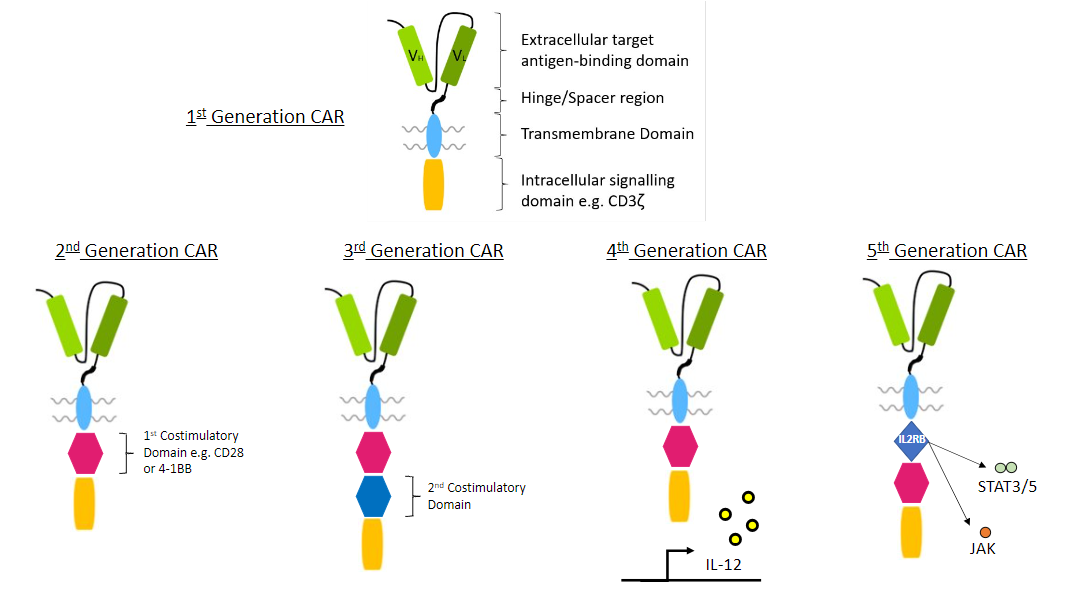
1st generation CAR
CARs are engineered receptors designed to modulate T cell receptors (TCRs) and consist of four main components:
(i) an extracellular target antigen-binding domain,
(ii) hinge or spacer region,
(iii) transmembrane domain, and
(iv) an intracellular signaling domain, e.g., CD3zeta
2nd generation CAR
Second-generation CARs additionally include one co-stimulatory molecule:
(i) an extracellular target antigen-binding domain,
(ii) hinge or spacer region,
(iii) transmembrane domain, and
(iv) a co-stimulatory domain, e.g., CD28 or 4-1BB
(v) an intracellular signaling domain, e.g., CD3zeta
3rd generation CAR
Third-generation CARs contain a second co-stimulatory domain:
(i) an extracellular target antigen-binding domain,
(ii) hinge or spacer region,
(iii) transmembrane domain, and
(iv) a first co-stimulatory domain, e.g., CD28 or 4-1BB
(v) a second co-stimulatory domain
(vi) an intracellular signaling domain, e.g., CD3zeta
4th generation CAR
The fourth generation of CARs are based on second-generation CARs (containing 1–3 ITAMs) paired with a constitutively- or inducibly-expressed cyokine (e.g., IL-12). These T cells are also referred to as T cell redirected for universal cytokine-mediated killing (TRUCKs).
Fourth-generation CAR T cells can activate downstream transcription factor to induce cytokine production after the CAR recognizes the target antigen.
5th generation CAR
Various 5th generation CARs are under development, which seek to improve the safety, specificity, tumour toxicity, and persistence of CAR-T cells.
In particular, while 4th generation CARs (TRUCKS) can drive the expression of one particular cytokine upon antigen binding, some 5th generation CARs have been developed with the aim of activating pathways that mediate downstream signalling of many cytokines, such as the JAK/STAT pathway.
To that end, some 5th generation CARs are also based on second-generation CARs, but incorporate an additional IL-2 receptor and a STAT3 recruitment motif. Upon target antigen binding, these CAR-Ts activate the JAK/STAT pathway, resulting in improved persistence and antitumour effects [1.
Patent filing strategies in an evolving field
Where a technical field is progressing and developing, it is normal to see patent filings tracking the output of scientific publications. However, each time an applicant files for an application for an “improvement” type invention, care needs to be taken to make sure that the criteria of novelty and inventive step in particular are fully satisfied.
It would be normal to assume that novelty will be present since the invention is an “improvement” over the earlier filing, e.g., a 2nd generation CAR with respect to a 1st generation CAR. If an applicant is only dealing with its own earlier patent in terms of relevant publications, it can be beneficial to consider filing any new improvement application just prior to the publication date of the earlier patent filing, i.e. just before the date 18 months from the earliest claimed priority date or filing date (a patent application is set to be published by this 18 month date from the earliest claimed priority date, or the filing date if no priority date is claimed). The earlier filed unpublished application will therefore only be citable for novelty, and not for inventive step.
However, inventive step is at issue, this will often need more detailed consideration. Sometimes it can be helpful to ask a series of questions designed to elicit whether or not there are sufficient grounds for inventive step to be present.
-
Does the invention solve a technical problem in a non-obvious way?
-
Does the prior art contain any pointers to the solution, or does the invention provide an unexpected technical effect?
In Europe, a wide range of technical effects can be used to support an inventive step. In addition to the typical measure of target cell killing, inventive step may also be acknowledged based on CAR-T persistence; unexpectedly low or high cytokine release compared to other CAR-Ts; specificity (i.e. a reduction in off-tumour effects); tolerability in vivo; unexpected therapeutic benefits in particular patient subsets; among many others. Conducting a review at the drafting stage of possible technical effects, and including at least a brief description of these in the application, can often reap rewards in Europe when it comes to subsequent prosecution.
For any advice about patent filing strategies in the field of cell based therapies, please contact Nick Bassil or your usual advisor at Kilburn & Strode LLP.