Congratulations to Susumu Kitagawa, Richard Robson and Omar M. Yaghi who were jointly awarded the 2025 Nobel Prize in Chemistry "for the development of metal–organic frameworks". In this article, we take a quick look at:
-
what metal–organic frameworks are,
-
how metal–organic frameworks might be used to tackle some of the most pressing environmental issues being faced today; and
-
trends in patent filings for metal–organic frameworks.
Alice is particularly interested in this topic because she completed her PhD research in this field. During her PhD, she was fortunate enough to attend the conference MOF2018 in Auckland, New Zealand, where she attended talks from the now Nobel Prize winners and got to learn first-hand about the development of this class of materials.
What are metal–organic frameworks?
Metal–organic frameworks (MOFs), discovered in the late 1980s1, are a class of chemical framework materials formed of metal ions or metal ion clusters (called nodes or secondary building units) coordinated with organic molecular linkers to form complex two-dimensional or three-dimensional frameworks.
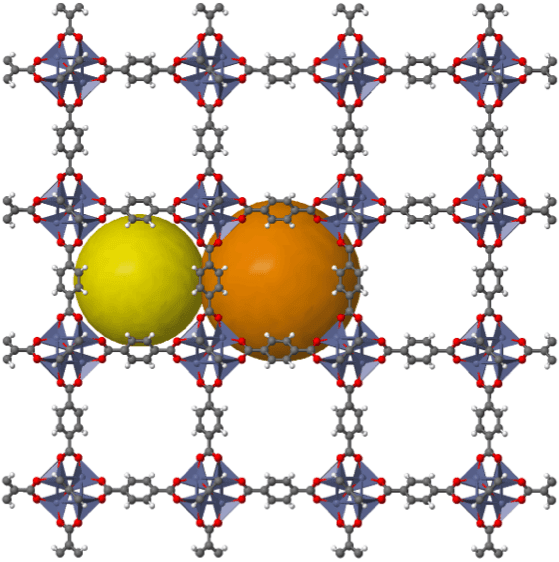
Source: https://www.chemtube3d.com/mof-mof5/
Through careful choice of the metal node and organic linker, complex and beautiful crystal architectures can be prepared. Precise control of the synthesis conditions can result in the same metal ions and organic linkers assembling to form different polymorphic crystal structures. Although the majority of MOFs are crystalline, many are also amorphous, and some can even be melted to form glasses (this was the focus of Alice’s PhD thesis).
Properties and applications of MOFs
One of the key properties of MOFs is their ultrahigh porosity.2 While zeolites have long been the go-to class of porous materials, MOFs offer significantly higher porosities. The use of organic molecules as linkers in MOFs results in scaffolding-like structures with large pores and enormous internal surface areas. A useful analogy is that many MOFs have an internal surface area larger than a football field! (For our friends in the US, we mean a soccer field, which is a lot bigger than a ‘football’ field.)
The exceptional porosity of MOFs has resulted in significant research into the ability of these materials to store molecules and separate gases, with many exciting potential applications being explored, including selective CO2 capture, encapsulating pharmaceuticals and trapping forever chemicals (PFAS).
MOFs from a patent perspective
The diverse potential applications of MOFs offer significant opportunities for patent protection.
This interesting article from Chemical & Engineering News provides a detailed discussion on patent activity in the field of MOFs and shows not only that the number of filings on MOFs has been increasing year on year, but also that MOFs have an extremely broad potential applicability across different technical fields. Key technical areas for patent protection are highlighted and include the use of MOFs as battery separator materials and for carbon dioxide capture, as well of course as the MOFs themselves and their chemical syntheses.
It is worth noting that many MOFs are polymorphic, meaning they can exist in more than one crystalline form. Different polymorphs of the same MOF can have significantly different properties including their porosity and their thermal stability. Crystalline polymorphs can be patented in Europe, allowing the possibility for patent protection to be extended beyond the original MOF disclosure. However, as discussed in our article Crystal clear? Five tips to patent polymorphs in Europe successfully, obtaining patent protection for polymorphs requires careful attention to inventive step, sufficiency and clarity requirements at the EPO.
Conclusion and outlook
The development of MOFs by Kitagawa, Robson and Yaghi has revolutionised the field of porous materials. As commercial applications continue to be developed, we expect to see increasing patent activity in this space and look forward to the environmental benefits that MOFs may bring.
For more information or advice on patenting chemical technologies, please contact Alice Bumstead, Jessica Smart, or your usual Kilburn & Strode advisor.
1. B. F. Hoskins and R. Robson, J. Am. Chem. Soc. 1989, 111, 15, 5962–5964 (https://pubs.acs.org/doi/abs/10.1021/ja00197a079)
2. H. C. Zhou, J. R. Long, and O. M. Yaghi, Chem. Rev., 2012, 112, 2, 673–674. (https://pubs.acs.org/doi/10.1021/cr300014x)