In early February, the first UK patient in Moderna’s Phase 1/2 MOBILIZE trial was administered a new cancer mRNA vaccine, mRNA-4359. In this article, we look at the technology behind mRNA-4359, the patents that cover that technology, and other ongoing trials in this exciting field.
Cancer mRNA vaccines: how do they work?
mRNA vaccines deliver stable forms of mRNA to the body. The delivered mRNA encodes specific proteins associated with the disease to be treated. For example, in the case of a melanoma mRNA vaccine, the mRNA might encode a protein known to be expressed by melanoma tumour cells. In the context of cancer, these types of proteins are often referred to as tumour-associated antigens (TAAs). Once delivered, the mRNA enables cells (such as dendritic cells) to produce those TAAs, and display them on the cell surface to initiate an immune response from T cells and B cells. The patient’s immune cells are then effectively programmed to recognise the TAA on cancer cells. In this way, mRNA vaccines can recruit the patient’s own immune system to fight cancer.
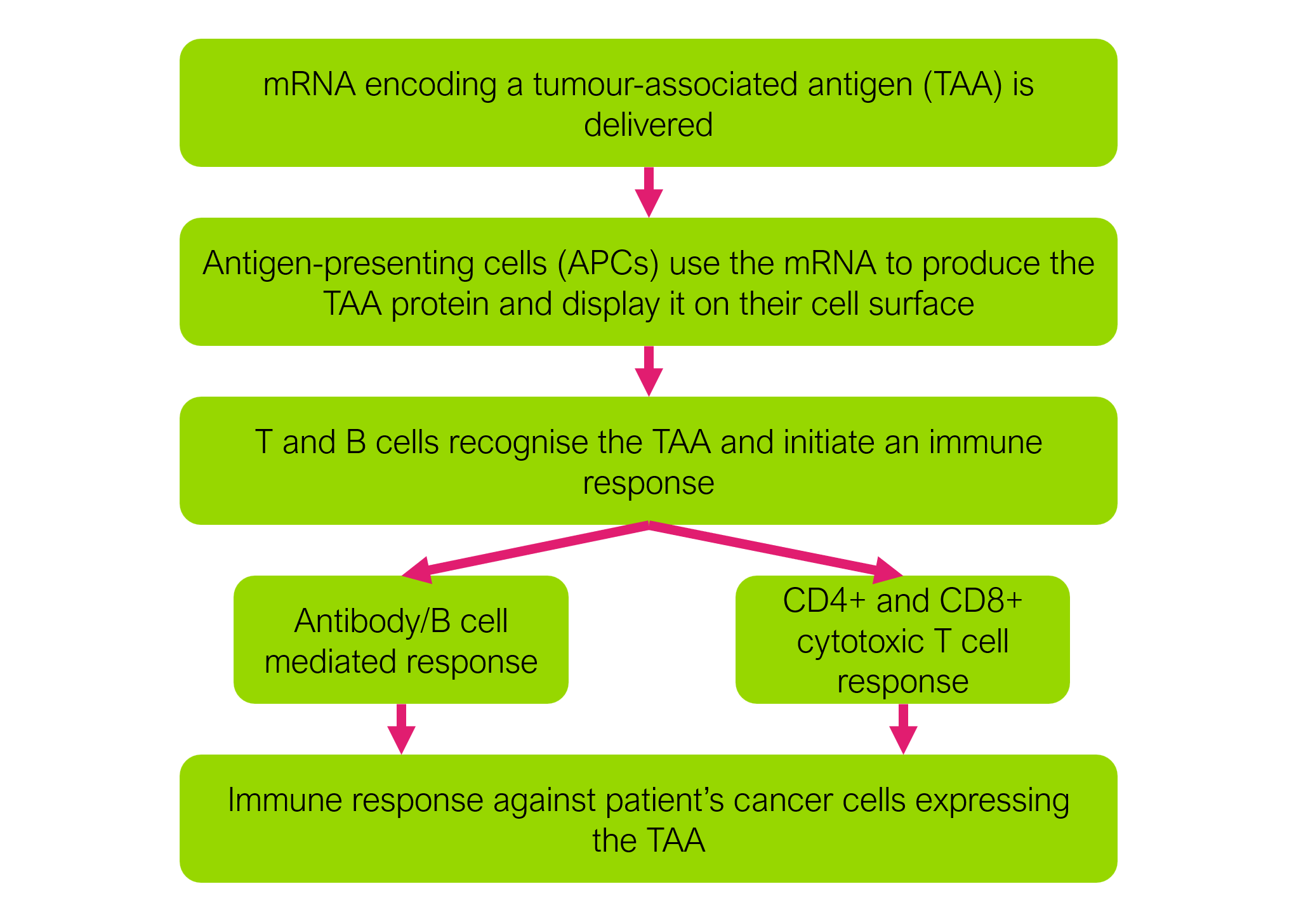
It is worth highlighting the difference between cancer vaccines and traditional vaccines for infectious diseases. Traditional vaccines, such as, for example, the measles vaccine, are prophylactic. This means that subjects are typically vaccinated before any infection occurs, priming the immune system so that the body can mount an effective immune response in the event the subject is ever exposed to the pathogen. In contrast, cancer vaccines are therapeutic. In other words, they are given to patients who already have cancer, in an effort to boost the immune system’s ability to fight the existing cancer.
Cancer mRNA vaccines: challenges and advantages
One of the challenges in providing an effective cancer vaccine is that many TAAs are not specific to the tumour alone, and are often expressed by non-cancerous, healthy tissue. Typically, T cells that bind such “self-antigens” are removed by the body’s own central and peripheral “tolerance” mechanisms. In a healthy individual, central and peripheral tolerance serve a protective function by eliminating those T cells that are self-reactive and could cause autoimmunity. But in cancer, these processes remove T cells which could potentially be useful in fighting the tumour cells. One of the challenges in cancer treatment, and in particular for cancer vaccines, is therefore to break this “immune tolerance” and activate those TAA-reactive T cells that remain[1]. In addition, if a TAA is expressed by healthy cells, a further challenge for cancer vaccines is to minimise the extent to which an immune response is directed to healthy tissue – so-called “on-target off-tumour” activity – which could lead to toxicity and unwanted side effects.
Despite these challenges, mRNA vaccine approaches have several advantages. Indeed, both BioNTech and Moderna used mRNA vaccine technology to develop their successful COVID-19 vaccines. mRNA vaccine approaches are generally considered less toxic than other approaches, and, unlike DNA vaccines, do not carry a risk of integration into the genome (so called insertional mutagenesis). “Off-the-shelf” mRNA cancer vaccines – those which encode a fixed set of TAAs and are not personalised to an individual’s tumour – are also a cost effective alternative to other types of cancer immunotherapy, such as CAR-T approaches, which often require the collection, ex vivo engineering, and re-administration of an individual patient’s T cells.
mRNA-4359: Moderna’s mRNA vaccine for solid tumours
Moderna’s mRNA vaccine “mRNA-4359” contains two types of mRNA, encoding two proteins known to be involved in immune suppression in cancer: PD-L1 and IDO.
PD-L1 is expressed on various cells types including activated T cells and B cells. Through interaction with its cognate receptor, PD-1, it is known to induce T cell anergy (tolerance to, and therefore failure to act against, an antigen) and immune suppression, leading to the inhibition of T cell proliferation and a reduction of cytotoxic activity and cytokine production. IDO is expressed by tumour cells as well as cells in the tumour microenvironment. It has been shown to impair cytotoxic effector T-cell function (the T-cell responsible for killing cancer cells) and to play an active role in acquired immune tolerance[2].
By redirecting a patient’s immune cells to target these immune suppressors, Moderna are hoping mRNA-4359 will show efficacy in a wide range of advanced solid tumours, including melanoma, non-small-cell-lung carcinoma (NSCLC), and bladder cancer. As well as investigating mRNA-4359 as a stand-alone therapy, the MOBILIZE trial will also look at the efficacy of a combination therapy with Merck/MSD’s incredibly successful anti-PD-1 antibody, pembrolizumab (Keytruda®).
Patent landscape
In terms of the patents and patent applications covering mRNA-4359, at least one, WO2023159197, has yet to enter the European regional phase as of February 2024. It is perhaps not surprising to note that several claims of WO2023159197 refer to “A lipid nanoparticle composition” that comprises the PD-L1 and IDO mRNAs. mRNA constructs are inherently unstable and suffer from poor in vivo delivery without specialised packaging and formulation. The use of lipid nanoparticles to deliver mRNA represents just one way of overcoming these challenges.
mRNA-4359 and methods of its production will no doubt also be covered by Moderna’s earlier platform applications, which have attracted attention recently following high profile infringement proceedings brought by Moderna against competitors BioNTech and Pfizer. Although those proceedings largely relate to alleged infringement by BioNTech’s COVID-19 vaccine, Comirnaty®, the outcomes may nonetheless be relevant to Moderna’s cancer vaccines, for example if any of Moderna’s platform patents are revoked or narrowed in scope.
In particular, Moderna’s European patent EP3590949 (the ‘949 patent) covers methods for synthesizing mRNAs in which uracil residues are replaced with N1-methyl-pseudouridine. The ‘949 patent also covers any mRNA in which 100% of the uracil residues are replaced with nucleotides comprising N1-methyl-pseudouridine. The N1-methyl-pseudouridine modification improves protein translation and is thought to underlie the higher efficacy of some mRNA vaccines compared to other, unmodified, mRNA vaccines[3]. Consequently, N1-methyl-pseudouridine modifications are expected to be present in a host of Moderna’s mRNA vaccines, including mRNA-4359, as well as in the mRNA vaccines of Moderna’s competitors. The ‘949 patent is currently being opposed at the EPO by eight opponents, with oral proceedings scheduled for 14 May 2024. These proceedings will no doubt be watched closely by many.
mRNA vaccines in cancer: future outlook
The MOBILIZE trial of mRNA-4359 is expected to continue until December 2027[4], so the potential efficacy of this vaccine won’t be known for some time. Moderna are also entering Phase 3 trials in the UK for their personalised mRNA cancer vaccine, mRNA-4157/V940[5]. Trialled in high-risk melanoma, the vaccine is capable of encoding up to 34 personalised antigens, dependent on the unique mutational signature of the individual’s tumour DNA sequence.
BioNTech are also trialling both “off-the-shelf” and personalised mRNA cancer vaccines. In December 2023, they began a Phase 2 trial of BNT113 in the UK[6]. BNT113 is being tested in HPV-positive head and neck cancer, and encodes two HPV antigens: serotype 16 oncoproteins E6 and E7. On the personalised side, BioNTech’s BNT122/RO7198457 can encode up to 20 personalised antigens and is currently being trialled in Phase 2 trials for colorectal cancer[7][8].
Elsewhere in Europe, CureVac began Phase 1 trials in June 2023 for their mRNA cancer vaccine candidate, CVGBM: a single mRNA encoding eight TAAs associated with glioblastoma[9].
With so many trials ongoing, and given the success of the mRNA vaccine approach in COVID-19, it is tempting to speculate that we may see positive results in cancer this decade. But mRNA vaccines still suffer from the same inherent challenges that many RNA-based therapies face, namely poor stability and the difficulty of in vivo delivery. Faced with these challenges, we expect to see plenty of innovation in the RNA packaging and formulation fields in the coming years. Lipid nanoparticles, nanoemulsions, polymer formulations, and exosome technology represent just some of the areas being explored, and we are excited to see how emerging technologies from these fields will allow mRNA cancer vaccines to realise their full potential.
Our team has considerable experience advising clients on patent matters in relation to technologies such as mRNA vaccines, RNA interference (RNAi), antisense oligonucleotides, non-coding RNA (ASO), CRISPR-based genome editing and LNP chemistry.
If you have a question relating to these technologies, please get in touch with one of our RNA experts: Andrea Hadfield, Beth Ormrod, James Cochrane, Jamie Atkins, Jessica Duncombe, Juliette Howarth, Oliver Lam, Samuel Bailey, Sarah Lau and Tom Leonard.
References:
[1] Hollingsworth RE & Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019. 4:7 https://doi.org/10.1038/s41541-019-0103-y
[2] Meireson A, Davos M, Brochez L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020. 11:531491. https://doi.org/10.3389/fimmu.2020.531491
[3] Morais P, Adachi H, Yu Y. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 2021. 9:789427. https://doi.org/10.3389/fcell.2021.789427
[4] https://bepartofresearch.nihr.ac.uk/trial-details/trial-detail?trialId=38730&location=&distance=
[5] https://bepartofresearch.nihr.ac.uk/trial-details/trial-detail?trialId=49363&location=&distance=
[6] https://bepartofresearch.nihr.ac.uk/trial-details/trial-detail?trialId=34170&location=&distance=
[7] https://bepartofresearch.nihr.ac.uk/trial-details/trial-detail?trialId=26015&location=&distance=
[8] https://investors.biontech.de/news-releases/news-release-details/biontech-expands-clinical-oncology-portfolio-first-patient-dosed
[9] https://www.curevac.com/en/curevac-doses-first-patient-in-phase-1-study-of-cancer-vaccine-candidate-for-surgically-resected-glioblastoma/